
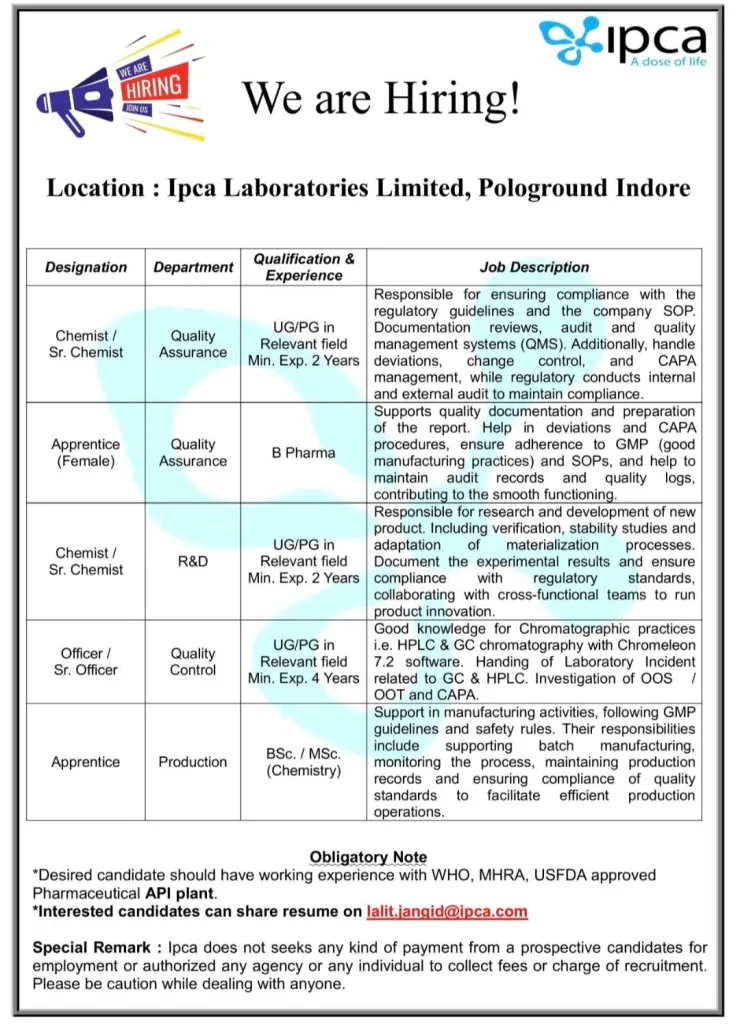
Ipca Laboratories Ltd
Location: Ipca Laboratories Limited, Pologround Indore
1) Department: Quality Assurance
Designation: Chemist / Sr. Chemist
Qualification & Experience:
UG/PG in Relevant field Min. Exp. 2 Years
Job Description:
Responsible for ensuring compliance with the regulatory guidelines and the company SOP. Documentation reviews, audit and quality management systems (QMS). Additionally, handle deviations, change control, and CAPA management, while regulatory conducts internal and external audit to maintain compliance.
2) Department: Quality Assurance
Designation: Apprentice (Female)
Qualification: B Pharma
Job Description:
Supports quality documentation and preparation of the report. Help in deviations and CAPA procedures, ensure adherence to GMP (good manufacturing practices) and SOPs, and help to maintain audit records and quality logs, contributing to the smooth functioning.
3) Department: R&D
Designation: Chemist / Sr. Chemist
Qualification & Experience:
UG/PG in Relevant field Min. Exp. 2 Years
Job Description:
Responsible for research and development of new product. Including verification, stability studies and adaptation of materialization processes. Document the experimental results and ensure compliance with regulatory standards, collaborating with cross-functional teams to run product innovation.
4) Department: Quality Control
Designation: Officer / Sr. Officer
Qualification & Experience:
UG/PG in Relevant field Min. Exp. 4 Years
Job Description:
Good knowledge for Chromatographic practices i.e. HPLC & GC chromatography with Chromeleon 7.2 software. Handing of Laboratory Incident related to GC & HPLC. Investigation of OOS / OOT and CAPA.
Department: Production
Designation: Apprentice
Qualification: BSc. / MSc. (Chemistry)
Job Description:
Support in manufacturing activities, following GMP guidelines and safety rules. Their responsibilities include supporting batch manufacturing, monitoring the process, maintaining production records and ensuring compliance of quality standards to facilitate efficient production operations.
Obligatory Note:
Desired candidate should have working experience with WHO, MHRA, USFDA approved Pharmaceutical API plant.
Interested candidates can share resume on lalit.jangid@ipca.com
Special Remark: Ipca does not seeks any kind of payment from a prospective candidates for employment or authorized any agency or any individual to collect fees or charge of recruitment. Please be caution while dealing with anyone.
To apply for this job please visit www.ipca.com.